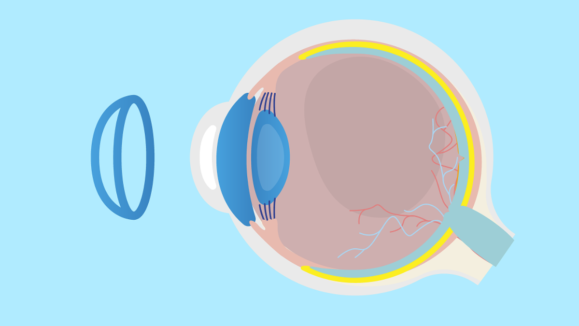
Multifocal Lenses Slow Myopia in Kids by Thickening Choroid, According to Study
A University of Houston study links lens use to biological changes that reduce axial length elongation. What’s going on behind…
Prevent Blindness Launches Patching and Amblyopia Awareness Campaign Tied to Disney/Pixar’s Elio
New amblyopia resources and videos support early detection of children’s vision disorders Ahead of the release of Disney/Pixar’s Elio, a…
popular posts
latest posts