Latest data shows TearCare delivers nearly two years of sustained dry eye relief after only two treatments.
The Phase III findings from Sight Sciences’ (California, United States) Sahara randomized controlled trial are now in, and the results are telling.
Published in Optometry and Vision Science, the data shows that after just two TearCare treatments in the first five months, patients achieved clinically significant gains in tear stability, gland function and symptom relief that lasted nearly two years, with most requiring no additional retreatment.1
READ MORE: Alcon Launches TRYPTYR in U.S. for Dry Eye Disease Treatment
The Sahara trial at a glance
The Sahara randomized controlled trial is one of the largest and most rigorous evaluations to date of a device-based therapy for dry eye disease associated with meibomian gland dysfunction (MGD). Conducted across 19 U.S. sites, Phase III enrolled patients with moderate-to-severe disease and followed them for more than two years.
The study was structured in three stages. In Phase I, TearCare localized heat therapy with manual gland expression was compared with cyclosporine ophthalmic emulsion (AbbVie’s Restasis). TearCare achieved superior improvements in tear stability and demonstrated non-inferior symptom relief.
In Phase II, patients originally assigned to cyclosporine crossed over to TearCare and experienced similar gains. Phase III extended follow-up for patients who had received two TearCare treatments, assessing the durability of benefits over the long term.
READ MORE: Stuart Therapeutics Unveils Phase III Results for Novel Dry Eye Candidate
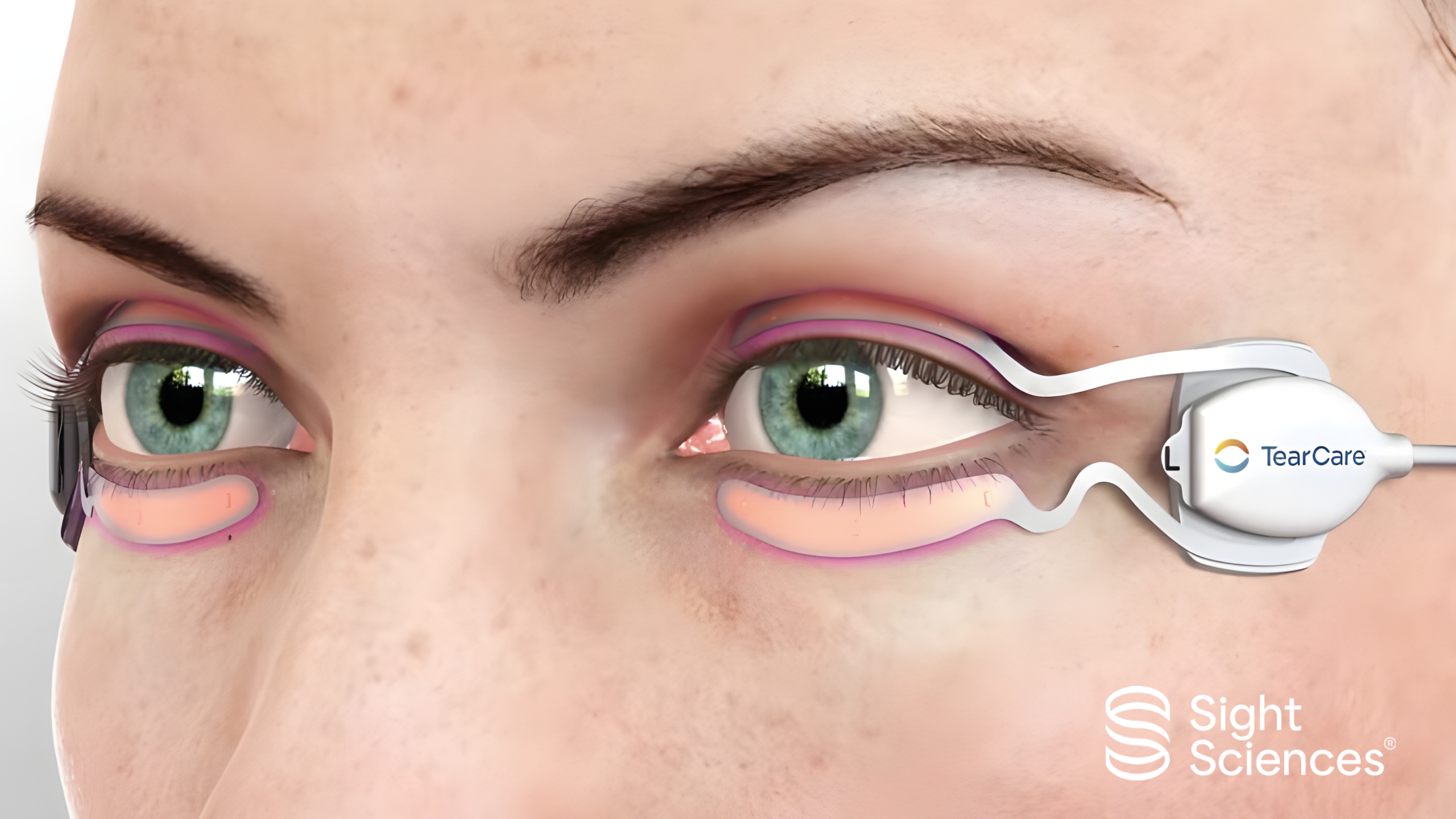
What is TearCare?
TearCare is a device-based therapy that delivers precisely controlled heat (45°C for 15 minutes) to the eyelids via SmartLids, followed by manual expression of the glands. This approach directly targets the obstructed meibomian glands that drive evaporative dry eye disease.
Unlike home remedies such as warm compresses, TearCare is designed to offer precise temperature control and professional clearance of the glands, ensuring both consistency and efficacy.
Key findings from the Phase III trial
- Improved tear film stability and gland function
At study entry, patients had unstable tears with an average TBUT of 4.4 seconds. After two TearCare sessions, TBUT improved to around 7 seconds and remained significantly above baseline through 24 months. Meibomian gland secretion scores, which nearly tripled after initial treatment, also stayed elevated throughout Phase II.1 - Rapid symptom relief
On the Ocular Surface Disease Index (OSDI), patients improved by about 18 points after the second treatment and maintained a benefit of roughly 14 points above baseline at two years. This exceeds the threshold for clinically meaningful improvement and translates into noticeable relief in day-to-day life.1
- Durable outcomes with minimal retreatment
Two-thirds of patients never required another TearCare session during Phase III. Among those who did, the median time to retreatment was eight months. Even after retreatment, improvements remained significantly better than baseline.1 - Strong safety profile:
Over 19 months, there were a small number of adverse events, none of which were linked to TearCare. No changes in visual acuity or eye pressure were observed, underscoring the safety of the procedure.1
READ MORE: Bausch + Lomb Phase IV Study Shows Rapid Symptom Relief with MIEBO for DED
Looking at the big picture
The Sahara Phase III trial offers some of the clearest evidence to date that an office-based, device-driven therapy can deliver sustained relief for patients with MGD-related dry eye. For clinicians, the durability of TearCare’s effects reduces the burden of frequent retreatment and supports its use as a frontline intervention. For patients, the results translate into the possibility of nearly two years of meaningful comfort after only two treatment sessions.
Perhaps most importantly, these findings could reinforce a shift in how dry eye disease can be managed: from short-term palliative care to long-lasting restoration of gland function. TearCare’s performance in the Sahara trial positions it as a practical, repeatable and safe solution that could reshape dry eye management for both providers and patients alike.
Editor’s Note: This content is intended exclusively for healthcare professionals. It is not intended for the general public. Products or therapies discussed may not be registered or approved in all jurisdictions, including Singapore.
Reference
- Hovanesian J, Ayres BD, Bloomenstein MR, et al. Durability of the TearCare treatment effect in subjects with dry eye disease: Stage 3 of the Sahara randomized controlled trial. Optom Vis Sci. 2025;102(8):495-504.