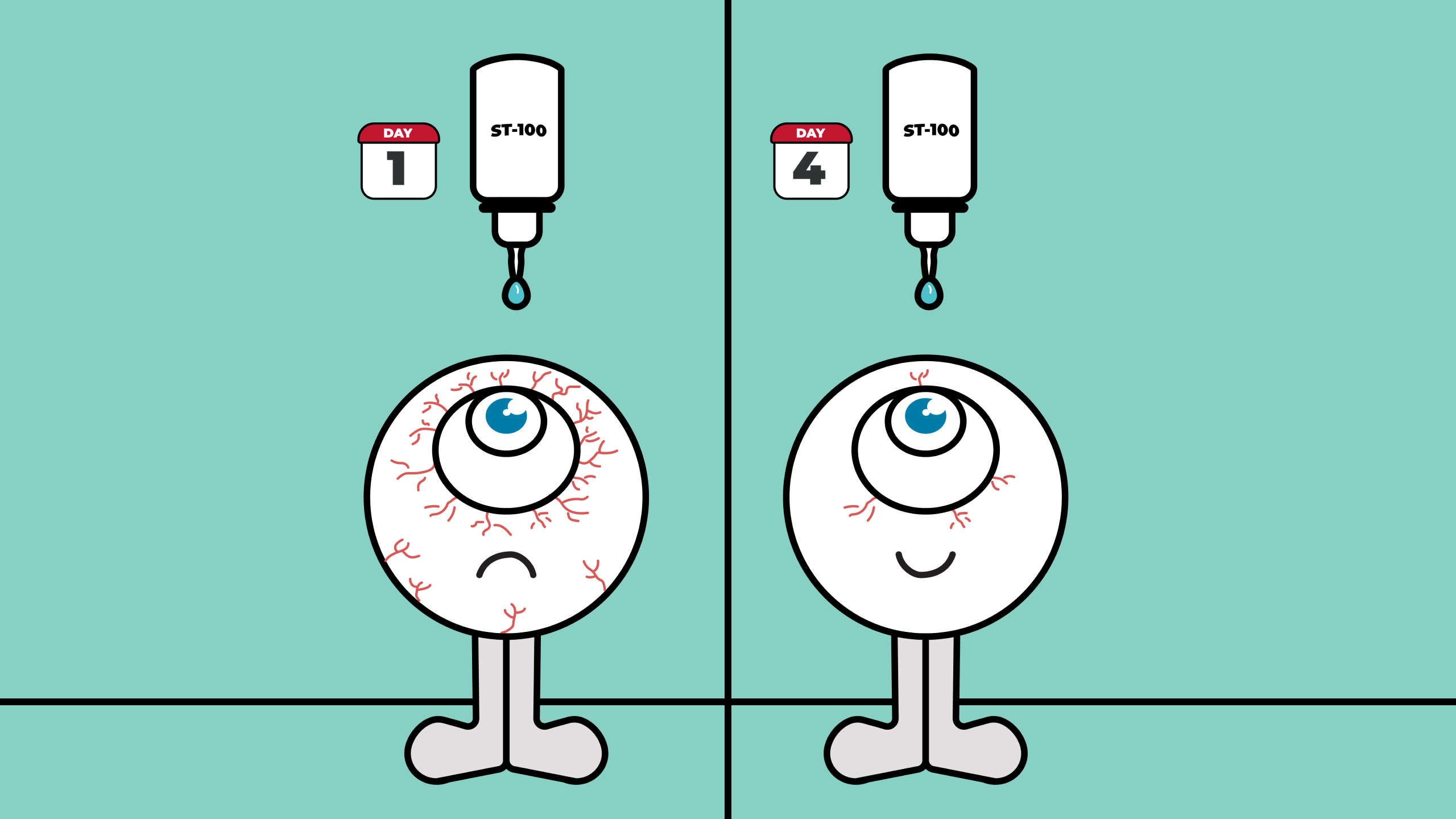
ST-100 missed its primary endpoint—but showed visible healing on the eye’s surface by Day 4.
Stuart Therapeutics (Florida, United States) has announced preliminary results from its Phase III clinical trial of ST-100 (vezocolmitide), an investigational treatment for dry eye disease (DED).
The trial did not meet its primary endpoint of improved tear production with statistical significance. But it did show early, clinically meaningful improvements in ocular surface health and visual function—a potential sign of promise for the new approach to dry eye disease management that ST-100 represents.
ST-100 targets ocular collagen repair
ST-100 is part of a new class of therapies that aim to restore the eye’s surface by repairing damaged collagen, rather than targeting inflammation or tear production directly.
This mechanism is based on PolyCol, Stuart Therapeutics’ proprietary platform using collagen mimetic peptides. The drug’s goal is to interrupt the cycle of tissue damage and inflammation by restoring structural integrity at the ocular surface.
Preclinical data for ST-100 have shown the compound supports cell health and function across several tissue types, including epithelial, neuronal and endothelial cells.1,2 It’s also been linked to reductions in oxidative stress and inflammation—key drivers in dry eye and other ocular diseases.3
READ MORE: Quenching Dry Eye with Dr. Tina Khanam
Inside the trial
The 29-day, randomized, double-blind, vehicle-controlled trial measured a variety of endpoints, including the Schirmer’s Responder Rate (SRR). Despite missing its primary endpoint of tear production improvement, ST-100 showed promising effects elsewhere, with rapid ocular surface healing and better visual function emerging in the early days of treatment.
READ MORE: Iolyx Announces Promising Phase II Results for ILYX-002 in Autoimmune DED
According to the study, statistically significant improvements in fluorescein staining were observed as early as Day 4 (>20%), with broader improvements noted by week 1. Visual function scores, another secondary measure, improved significantly by Day 2, suggesting rapid symptomatic relief.
Stuart Therapeutics President and CEO Eric Schlumpf said: “While additional study is required, the underlying data and the clinically meaningful results from this trial strongly suggest that ST-100, as the first drug candidate in a novel therapeutic class, can address the critical unmet needs in dry eye disease.”
Dr. Jodi Luchs, chief medical officer and practicing ophthalmologist, added: “The results suggest rapid ocular surface healing and treatment effects that appear to be considerably greater than those seen with currently approved dry eye disease therapeutics.”
WATCH NOW: How to Tackle Dry Eye
Additional trials and FDA pathway
Looking ahead, Stuart Therapeutics plans to design a follow-up clinical trial based on these latest results and will consult with the U.S. Food and Drug Administration (FDA) to finalize the path toward regulatory approval.
While more data will be needed before ST-100’s clinical potential can be confirmed, the results point to its early symptom improvements and distinct mechanism of action as indicators worth further exploration.
Editor’s Note: See the full Stuart Therapeutics press release here. This content is intended exclusively for healthcare professionals. It is not intended for the general public. Products or therapies discussed may not be registered or approved in all jurisdictions, including Singapore.
References
- Baratta RO, Del Buono BJ, Schlumpf E, Ceresa BP, Calkins DJ. Collagen Mimetic Peptides Promote Corneal Epithelial Cell Regeneration. Front Pharmacol. 2021;12:705623.
- Wareham LK, Holden JM, Bossardet OL, et al. Collagen mimetic peptide repair of the corneal nerve bed in a mouse model of dry eye disease. Front Neurosci. 2023;17:1148950.
- Ribeiro M, Pasini S, Baratta RO, Del Buono BJ, Schlumpf E, Calkins DJ. Collagen Mimetic Peptides Promote Adherence and Migration of ARPE-19 Cells While Reducing Inflammatory and Oxidative Stress. Int J Mol Sci. 2022;23(13):7004.